ABOUT ANASCORP
Clinical Data1
The efficacy of ANASCORP was assessed in multiple studies:
- 1 prospective double-blind, randomized, placebo-controlled study
- 4 open-label studies
- 1 retrospective study in various treatment settings in the United States, where scorpion envenomation is common

Patient Population1
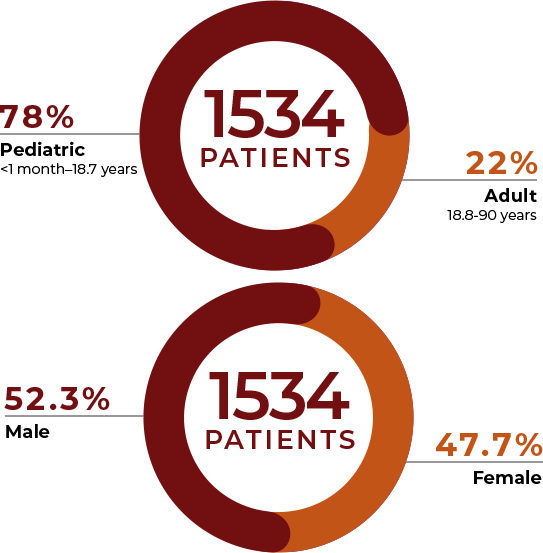
A total of 1534 patients ranging from less than 1 month to 90 years old were treated. The majority (78%, 1204/1534) were pediatric, ranging from less than 1 month to 18.7 years of age. Male (52.3%) and female (47.7%) patients were equally represented.
Treatment Success1
Treatment success was determined by resolution of clinically important signs of scorpion envenomation within 4 hours of starting infusion.
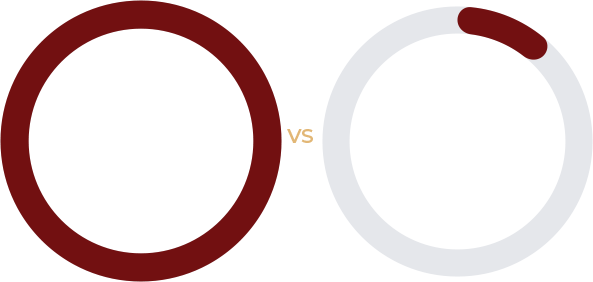
The randomized placebo-controlled study enrolled 15 subjects, 8 to the ANASCORP-treated group, and 7 to placebo. The symptom resolution success rate was 100% for the ANASCORP-treated and 14.3% for the placebo group.
Symptom Resolution Success Rate at 4 Hours
Retrospective Analysis1
A retrospective hospital chart review provided historical data from envenomated patients (n=97) who did not receive antivenom but were treated with sedatives and supportive care for symptoms of envenomation. These data were used as a historical control for expected outcomes in the absence of antivenom treatment.
The historical controls were pediatric patients admitted to 2 pediatric intensive care units between 1990 and 2003 for the treatment of scorpion envenomation with supportive care only. The proportion of patients that required intensive care support 4 hours after intensive care unit admission, and the overall duration of the intensive care support requirement, were calculated.
Results1
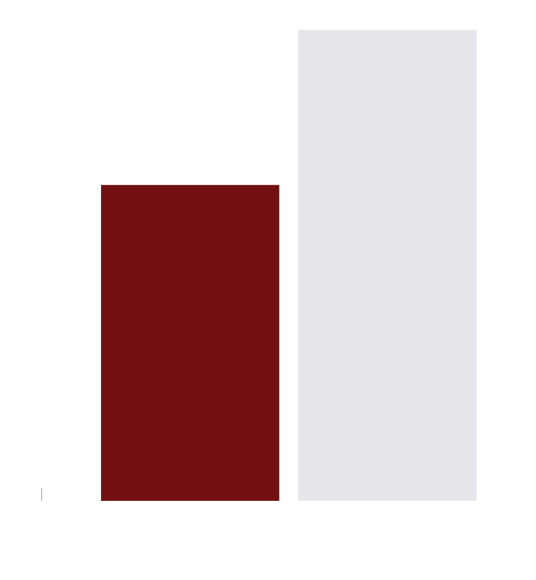
In 1396/1534 patients, the mean time from start of ANASCORP infusion to resolution of clinical signs and symptoms of envenomation was 1.42 hours (0.2 to 20.5 hours). Pediatric patients generally experienced a slightly faster time to resolution (1.28 ± 0.8 hours) compared with that of adult patients (1.91 ± 1.4 hours). The time to resolution of symptoms was not affected by use of sedatives (474 patients who received sedatives resolved in 1.49 ± 1.1 hours and 922 patients who did not receive sedatives resolved in 1.38 ± 0.9 hours).
Mean Time to Symptom Resolution
1. ANASCORP [centruroides (scorpion) immune F(ab’)2 (equine)] injection Prescribing Information. Rare Disease Therapeutics, Inc.; Franklin, TN. August 2022.
INDICATION
ANASCORP® [centruroides (scorpion) immune F(ab')2 (equine) injection] is an equine-derived antivenom indicated for treatment of patients with clinical signs of scorpion envenomation.
IMPORTANT SAFETY INFORMATION
INDICATION
ANASCORP® [centruroides (scorpion) immune F(ab')2 (equine) injection] is an equine-derived antivenom indicated for treatment of patients with clinical signs of scorpion envenomation.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Severe hypersensitivity reactions, including anaphylaxis, may occur with ANASCORP. Close patient monitoring for hypersensitivity reactions and readiness with intravenous therapy using epinephrine, corticosteroids, and diphenhydramine hydrochloride is recommended during the infusion of ANASCORP. If an anaphylactic reaction occurs during the infusion, terminate administration at once and administer appropriate emergency medical care. Patients with known allergies to horse protein are particularly at risk for an anaphylactic reaction. Patients who have had previous therapy with ANASCORP or another equine antivenom/antitoxin may have become sensitized to equine protein and be at risk for a severe hypersensitivity reaction.
Delayed Allergic Reactions
(Serum Sickness) Monitor patients with follow-up visit(s) for signs and symptoms of delayed allergic reactions or serum sickness (e.g., rash, fever, myalgia, arthralgia), and treat appropriately if necessary. Eight out of 1,534 (0.5%) patients in the clinical trials exhibited symptoms suggestive of serum sickness.
Transmissible Infectious
Agents ANASCORP is made from equine (horse) plasma, it may therefore carry a risk of transmitting infectious agents, e.g., viruses.
Reaction to Cresol
Trace amounts of cresol from the manufacturing process are contained in ANASCORP. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient.
ADVERSE REACTIONS
The most common adverse reactions observed in ? 2% of patients in the clinical studies for ANASCORP were: vomiting, pyrexia, rash, nausea and pruritus.
See Full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ANS-ISIF-001
ANASCORP® [centruroides (scorpion) immune F(ab')2 (equine) injection] is an equine-derived antivenom indicated for treatment of patients with clinical signs of scorpion envenomation.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Severe hypersensitivity reactions, including anaphylaxis, may occur with ANASCORP. Close patient monitoring for hypersensitivity reactions and readiness with intravenous therapy using epinephrine, corticosteroids, and diphenhydramine hydrochloride is recommended during the infusion of ANASCORP. If an anaphylactic reaction occurs during the infusion, terminate administration at once and administer appropriate emergency medical care.
Patients with known allergies to horse protein are particularly at risk for an anaphylactic reaction. Patients who have had previous therapy with ANASCORP or another equine antivenom/antitoxin may have become sensitized to equine protein and be at risk for a severe hypersensitivity reaction.
Delayed Allergic Reactions (Serum Sickness)
Monitor patients with follow-up visit(s) for signs and symptoms of delayed allergic reactions or serum sickness (e.g., rash, fever, myalgia, arthralgia), and treat appropriately if necessary. Eight out of 1,534 (0.5%) patients in the clinical trials exhibited symptoms suggestive of serum sickness.
Transmissible Infectious Agents
ANASCORP is made from equine (horse) plasma, it may therefore carry a risk of transmitting infectious agents, e.g., viruses.
Reaction to Cresol
Trace amounts of cresol from the manufacturing process are contained in ANASCORP. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient.
ADVERSE REACTIONS
The most common adverse reactions observed in ≥ 2% of patients in the clinical studies for ANASCORP were: vomiting, pyrexia, rash, nausea and pruritus.
Please see complete Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com, or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ANS-ISIF-001

2550 Meridian Blvd. Suite 150
Franklin, TN 37067
To order ANASCORP, call:
1-844-4RareTx (1-844-472-7389)
ANASCORP is exclusively distributed by:
AnovoRx www.anovorx.com
![ANASCORP® [centruroides (scorpion) immune F(ab')? (equine) injection]](https://www.anascorp-us.com/wp-content/uploads/2024/05/AnascorpLogo_color-tall.png)